Carbon capture, utilization, and storage (CCUS) in landscape architecture (part 2) - Carbon release and carbon sequestration
Professor of Landscape Ecology, Department of Landscape Architecture, Chinese Culture University, Taiwan
This article is the second of a series of four articles that re-examine the multidimensional aspects of carbon cycles. In the previous installment of this series, we discussed the different types of carbon cycles (carbon positive cycles, net-zero carbon cycles, and carbon negative cycles), how carbon storage is linked to organism lifespan and landscape maintenance, and life-cycle carbon assessments. In this second installment, we shall discuss how carbon storage is affected by decomposition rates and various means of extending the lifetime of carbon storage. In the third and fourth installments, we shall discuss how we may creatively make use of carbon cycles for the benefit of humans, and draft a carbon-healthy framework for landscape planning, design, management, and what Landscape Associations can do.
Carbon sequestration and the factors that affect carbon release
As detailed in the last article, the concentration of atmospheric CO2 is determined by the balance between carbon capture, in which atmospheric CO2 is taken out of the air and fixed as non-gaseous form, and carbon release, where carbon is released back into the air as CO2. If the storage time of the fixed-carbon is short, this flow in and out of gaseous form would be continuous, and a net-zero carbon cycle would be heavily dependent on constant and rapid carbon capture. If, instead, carbon is captured and then stored in non-gaseous form for a significant length of time, it would effectively enhance the effect of each carbon capture, and may potentially lower the total global atmospheric CO2 concentration. This capture and long-term storage of carbon is called carbon sequestration. The length of this storage period is affected by organism lifespan and the rate of carbon release through decomposition and burning. Burning releases carbon immediately. Decomposition, on the other hand, may take from a few hours to decades, depending on several factors that affect the rate of decomposition. Conditions that slow decomposition helps carbon sequestration.
Temperature and soil moisture: Decomposing bacteria and fungus are usually more active around 25-30 °C and a soil moisture level of 40-60%. Therefore, in general, the release of carbon from dead organisms back into the air is slower, and hence more potential for carbon sequestration, in cooler or drier climates.
Oxygen availability: Aerobic decomposers, who require oxygen to grow, work much faster than anaerobic decomposers. Therefore, turning the soil helps aerobic microbes, whereas water-logged environments such as wetlands often inhibit aerobic decomposition.
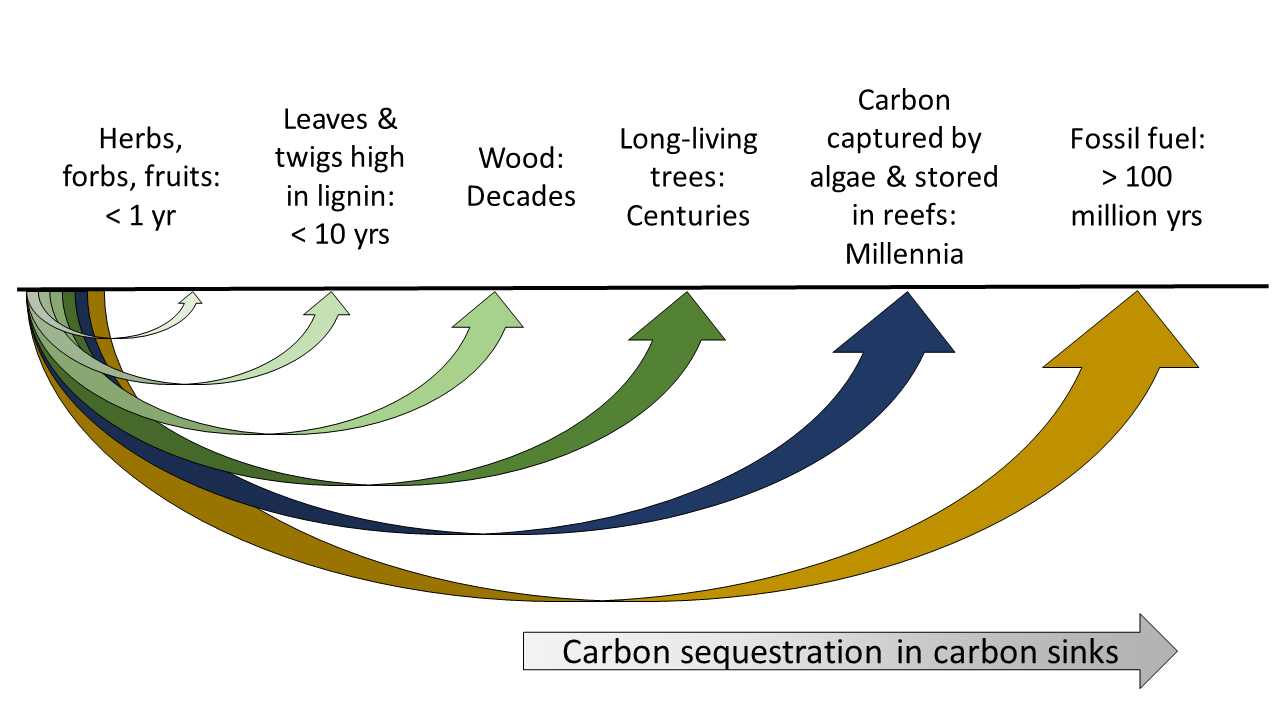
Nitrogen-lignin content of the organic material: Bacteria prefer organic materials with higher nitrogen content and lower lignin content (e.g. wood). This is why milk and meat start to go bad a few hours out of the refrigerator, fruit and vegetables may take a few weeks to decompose, whereas woody materials may take decades to rot. Therefore, herbs decompose faster than woody plants, leaves of woody plants decompose faster than branches, and branches decay faster than wood.
Presence of intermediary decomposers: Organic material with higher lignin content require intermediary decomposers such as insects (e.g. termite) and fungi to break the material down into smaller bits before earthworms and bacteria can further the decomposition process. This is why adding worms to a compost helps the decomposition process whereas controlling termite and fungus preserves timber longer.
Carbon sinks: Where effective carbon sequestration take place
Carbon sinks are carbon deposits in which carbon is entering the storage at a rate much faster than it is exiting. The more carbon sinks are created or maintained, the higher our chances of achieving the ultimate goal of a global reduction in atmospheric CO2. There are several potential types of carbon sinks:
Certain photosynthesizing organisms have very long lifespans or build structures that last for a long time, allowing them to become carbon sinks. Long-living trees, for example, may live for centuries to thousands of years. The crustose coralline algae also stores carbon by building reefs, both independently to form coralline algae reefs or by cementing coral reefs to build stronger structures.
Organic material may also extend its lifespan through the food chain. For example, organic carbon may first be stored in grass, then transformed to a rabbit, then to a hawk. This extends the carbon storage life-span from less than a year in an annual herb to perhaps 20 years through the rabbit and hawk. However, for this extension to be significant enough to be considered carbon sequestration, it usually requires a much longer period. A good example would be carbon captured by micro-algae and other phytoplanktons that are transferred and stored in various forms of reefs through corals and oysters. Phytoplanktons typically live and retain carbon for only a few days; by being eaten and then fixed in coral reefs or oyster reefs, however, they may be stored for centuries to millennia.
Soil carbon sinks also form when decomposition in the soil is slow, such that organic matter is stored in soil in the form of humus before decomposition is complete. It is estimated that of the total C stored in terrestrial ecosystems (approximately 3170 gigatons), nearly 80% (2500 GT) is found in soil. Because decomposition rates are slower in cooler climates, the largest carbon sinks per area are in the cooler climates.
Carbon sinks are also formed when significant amounts of organic materials enter a condition in which decomposition is arrested. One such example is wetlands, where the anaerobic conditions in the water significantly slow decomposition because the bacteria that are more effective at decomposition require oxygen. Peatlands, for example, are considered carbon sinks because they store a significant amount of organic material that have been stalled in their decomposition process. Sea beds are another significant carbon sink because the organic materials that have accumulated at the bottom of the ocean are also preserved by low oxygen levels.
Decomposition may also be slowed through the use of artificial preservation. Timber treatment and preservation, for example, extends the lifespan of the woody material, and keeps carbon out of the air for that period of time.
Geological carbon sequestration happens when the carbon sink is old enough to be buried in the geological layers. One such example is fossil fuels. The energy contained in these fossil fuels are actually carbon captured and stored by plants millions of years ago, and have been kept out of the air for millions of years. Burning fossil fuels releases the carbon that has been kept away from the atmosphere for millions of years back into the air, significantly perturbing the carbon balance.
Application by Landscape Architects
Burning releases carbon back into the air immediately. Landscape projects can help reduce carbon emissions by reducing management activities that involve burning and specifying practices with low energy consumption, energy efficiency, and use of green energy.
Reefs and ocean sediments store carbon for centuries to millennia. Landscape projects that involve coastal areas should map and plan to conserve coral, algal, and oyster reefs, and apply construction methods that avoid disturbing sea beds. Landscape designs and projects can also specify ways to recycle oyster shells to help grow new oysters and bolster a new carbon sequestering cycle.
Disturbing soils and draining wetlands accelerate the decomposition process. Carbon captured by plants decades to centuries ago and stored since are released back into the atmosphere. Landscape projects should therefore specify the conservation and minimal disturbances to soil and wetlands.
Organic materials richer in nitrogen such as fruits, vegetables, and lawn clippings decompose faster and are not an effective strategy for retaining carbon. Composts that induce faster decomposition, on the other hand, facilitate nutrient recycling. This accelerates the release of nitrogen and other nutrients for the use of other plants. So specifying materials with a short carbon cycle, such as fallen leaves, fruits, and lawn clippings for on-site recycling or composting is a better carbon strategy.
Herbicides alter microbial health of the soil. When the recycling of lawn clippings is intended, herbicides should be prohibited.
Applying biochar to soil further helps soil retain nutrients. Specifying the application of biochar recycled from agricultural and food wastes helps facilitate the next carbon cycle.
To prepare for forthcoming carbon assessment needs, each climate region should start estimating how much carbon soils sequester in different types of greenspaces.

